TMS Information for Patients
-
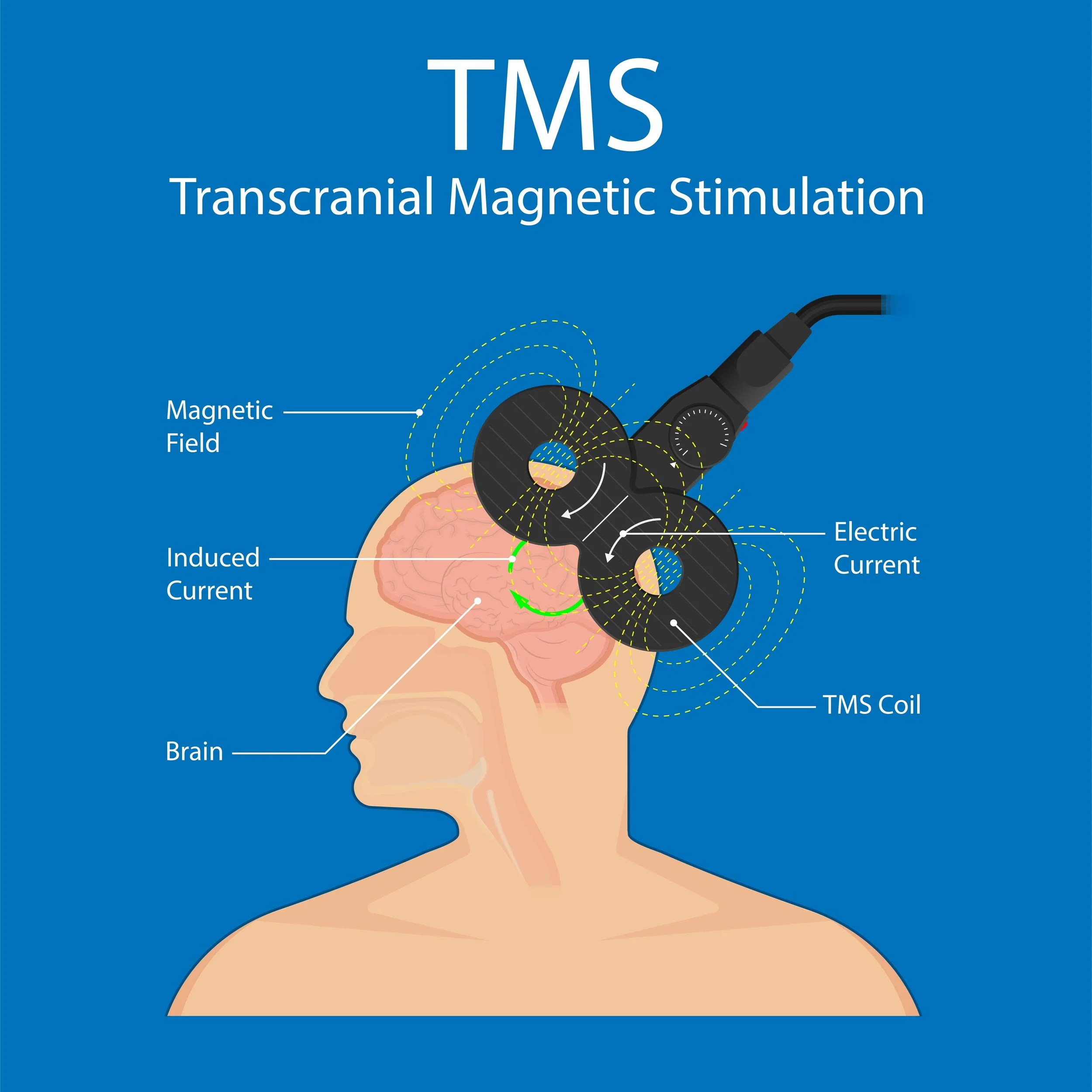
TMS stands for Transcranial Magnetic Stimulation. TMS is a non-invasive treatment that uses a magnetic field to stimulate nerves in the brain.
-
FDA indications for TMS include:
Major Depressive Disorder
OCD
Smoking cessation
Most insurance carriers will cover TMS treatment for MDD and OCD.
TMS can also be used to treat anxiety disorders like Generalized Anxiety Disorder.
-
Common side effects include:
Headache
Irritation near magnet placement
lightheadedness
-
Seizure
Mania
Hearing loss
-
TMS is typically conducted in an outpatient setting like an office. A typical course is 5 days a week for 36 treatments. (This may be different for an accelerated schedule which is explained below) An individual treatment can take anywhere between 3.5 to 40 minutes depending on the type of TMS machine being used, the indication and the individual settings of the machine.
The TMS machine can be loud so it is recommended that patients wear ear plugs to minimize the risk of hearing loss. There are no driving restrictions so patients can drive themselves to and from treatment. Unlike ECT, TMS has no cognitive side effects and there is no anesthesia required.
-
Only a trained and experienced physician can tell you if you are a good candidate for TMS. Having said this, TMS may be appropriate for you if you are finding it difficult to control your depression symptoms with more traditional treatments like medications, psyhotherapy, lifestyle modifications etc.
-
Patients with the following should avoid TMS:
Seizure history
Implanted metal in head, neck or face
Cochlear implants
Cardiac pacemakers, vagal nerve stimulators or medication pumps
Patients actively abusing substances
-
The data indicate that TMS is not as effective as ECT but has a much more favorable side effect profile.
no anesthesia
no cognitive side effect
no driving restrictions
no systemic effects (GI upset, sexual side effects, etc. like some people can get from medications)
reduced time off from work for above mentioned reasons
-
TMS Information for Clinicians
-
A TMS machine works by sending an electrical current through a wire coil which produces a magnetic field. The coil (magnetic field) is placed next to the patient’s head in specific areas which has an induction effect on the neurons. This magnetic field causes the neurons in the brain to depolarize which can have a stimulatory or inhibitory effect based on the anatomic location and machine settings.
-
There are different targets that have different effects on the brain. One of the most common targets is the dorsal lateral prefrontal cortex on the left side of the brain. This area has been implicated in depression and is the target for many treatment protocols. Please see diagram below.
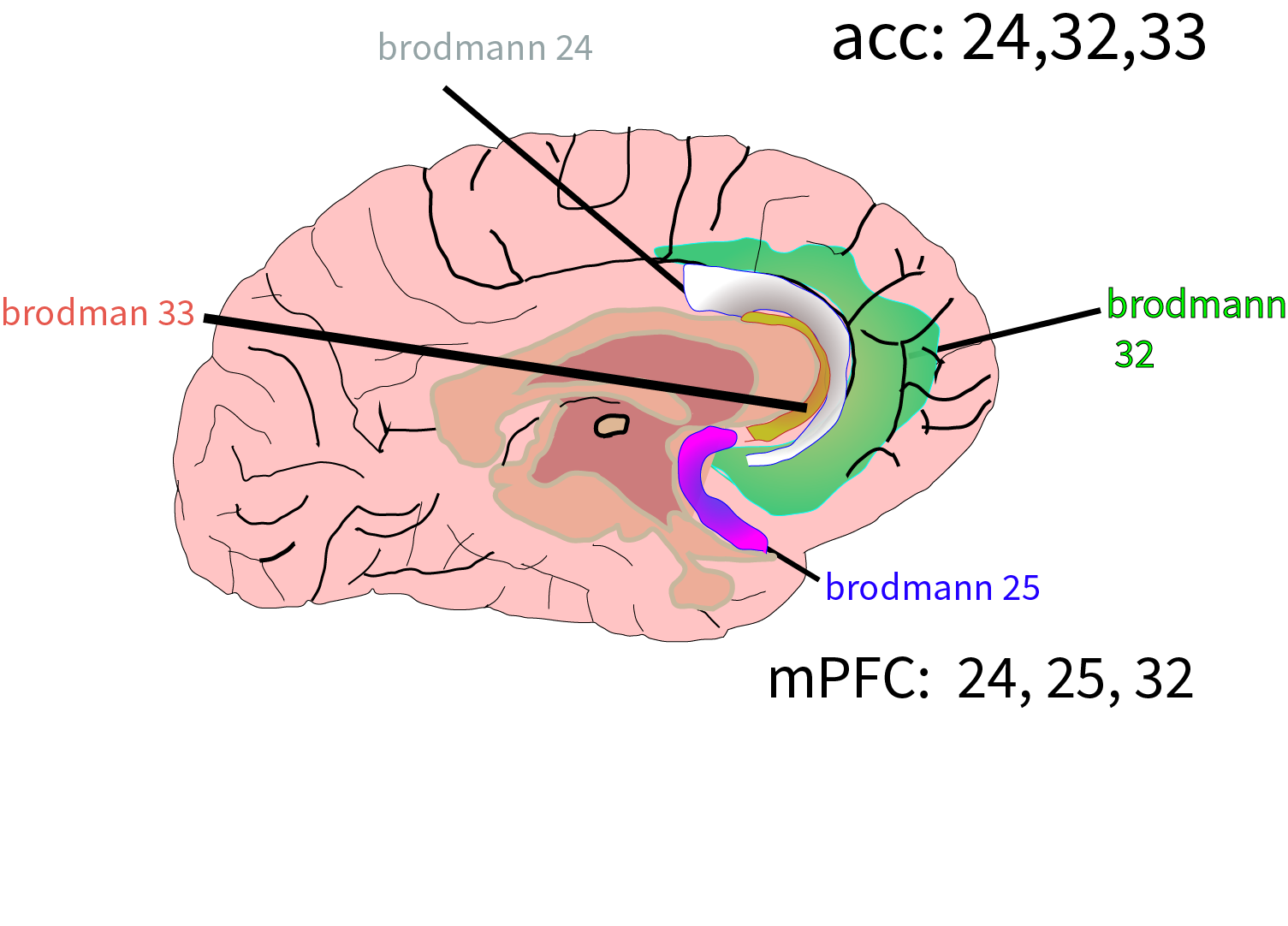
A common target for OCD utilizing deep TMS is the anterior cingulate cortex (aCC) and the medial prefrontal cortex (mPFC) as illustrated below. It takes a larger magnetic field to reach these areas as they are deeper in the brain.
-
MT stands for motor threshold. It is a scalar measure denoted as a percentage of the magnetic field strength needed to illicit a motor evoked potential (MEP) from the contralateral abutuctor policies brevis (thumb) when applied to the contralateral motor strip.
Most TMS protocols aim for a “dose” of 110-120% MT.
SAINT has an MT of 90%
-
5.5 cm method: This method involves stimulating the motor strip causing isolated movement in the contralateral abductor policies bravis muscle. (Thumb) We then move the magnet 5.5 cm anterior from that point.
Beam method: This method involves taking three different measurements and calculating the DLPC location from those measurements. The measurements include ear tragus to tragus, nasion to inion, and head circumference. These measurements are put into a formula which gives two numbers. The first number is the distance from the centerline that should be marked. (on a patient cap) Then the second number is the distance from the vertex of the head to the first marked point. Beam method formula can be found here.
10-20 method: Involved EEG leads which can be time and labor intensive. This is usually reserved for research settings.
Anatomic MRI: This method involves a patient undergoing a brain MRI with a special stereotactic headgear that can be used as a reference point. The patient then wears this head gear in a treatment room where a special camera can determine the exact location of the DLPC based on the MRI data. This is a costly method that does not necessarily lead to better outcomes. Li et al. 2020 concluded, “Left prefrontal piTBS monotherapy is effective for the treatment of recurrent depression, and the MRI-guided method of coil targeting is not better than the standard method.”
Functional MRI: It is hypothesized that depression arises from aberrantly functioning brain networks. Hadas et al. 2019 found “The findings of this study further implicate left DLPFC-SGC effective connectivity and SGC excitability in the pathophysiology of MDD and treatment with rTMS. These findings suggest that DLPFC-SGC connectivity may be a marker of rTMS treatment responsiveness.” This is the method utilized in the saint protocol.
For further reading on the various methods please refer to Zhang et al. 2021.
-
Figure eight coil
H-Coil
Double Cone Coil
There are other types but these are the most commonly used in FDA approved TMS treatment devices. One thing to consider is the trade off between increased magnetic field size like the H coil (able to penetrate deeper brain structures) and decreased target specificity. As your magnetic field gets bigger it is less focused and more broad.
For further technical information on coil types you may review Deng et al. 2016.
What are the different types of TMS?
-
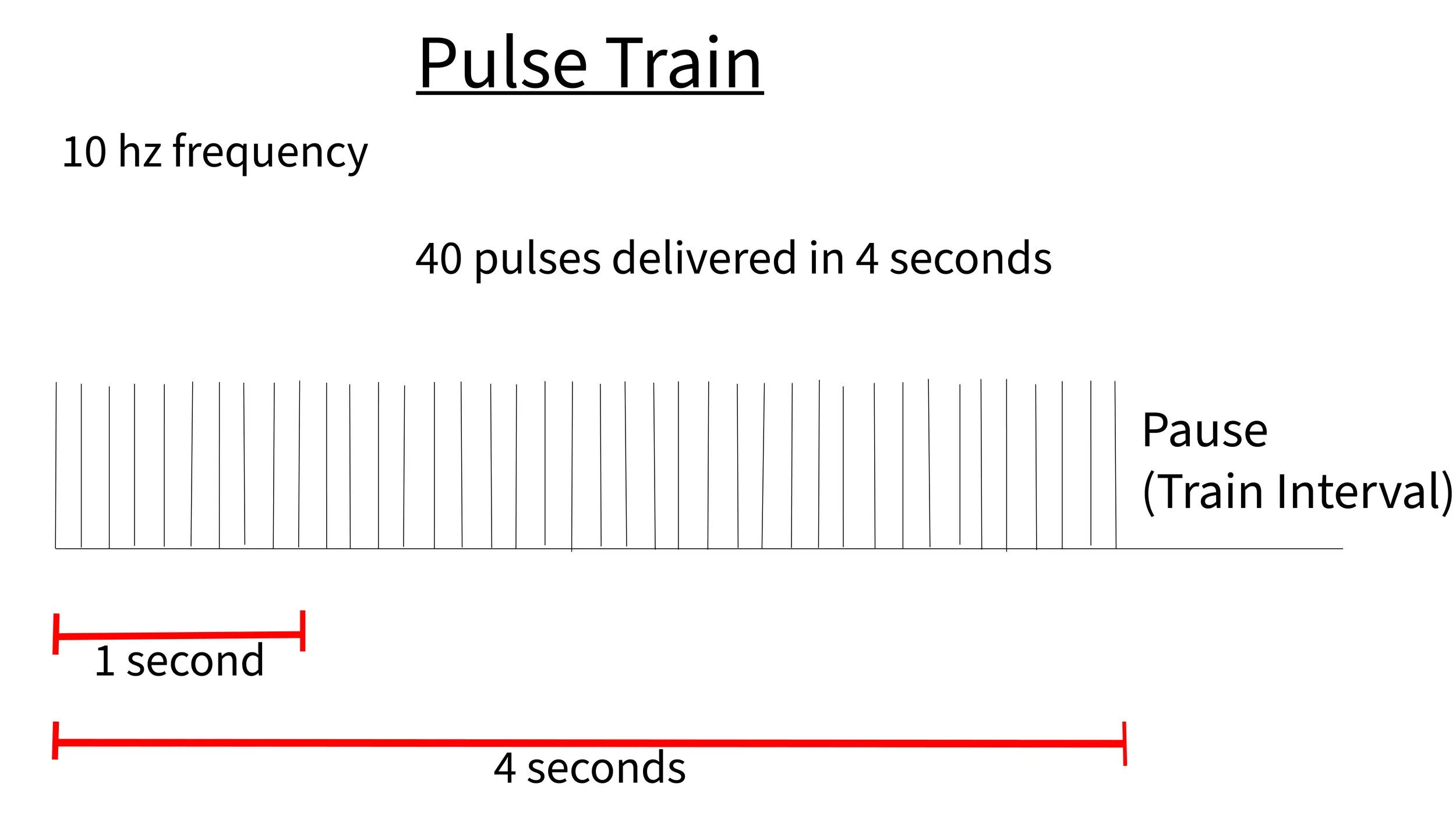
rTMS: This stands for “repetitive” TMS. It is the type of TMS that you are most likely to find in a clinical setting. This type of TMS can be used to treat depression and anxiety. This is typically delivered using a figure 8 coil magnet. rTMS involves giving a series of pulses, at a certain intensity, at a certain frequency for a certain duration of time. This set of pulses is often referred to as the “train.” The trains are followed by a rest period that can vary in duration.
rTMS can be delivered with:
High frequency: This is often referred to as “standard TMS.” (> 1 pulse per secnd) Typically delivered over Left Dorsal Lateral Prefrontal Cortex (lDLPC). This tends to have a stimulatory effect on the targeted area.
A typical treatment with high frequency left sided DLPC targeting will have 3000 pulses per session. This will typically involve 10 pulses per second (10 hz) at 100-120% MT (strength of magnetic field) with a pulse train lasting 4 seconds. A treatment session usually consists of 75 trains spaced approximately 10-50 seconds apart. This means the treatment will take approximately 17-54 minutes depending upon the machine settings.
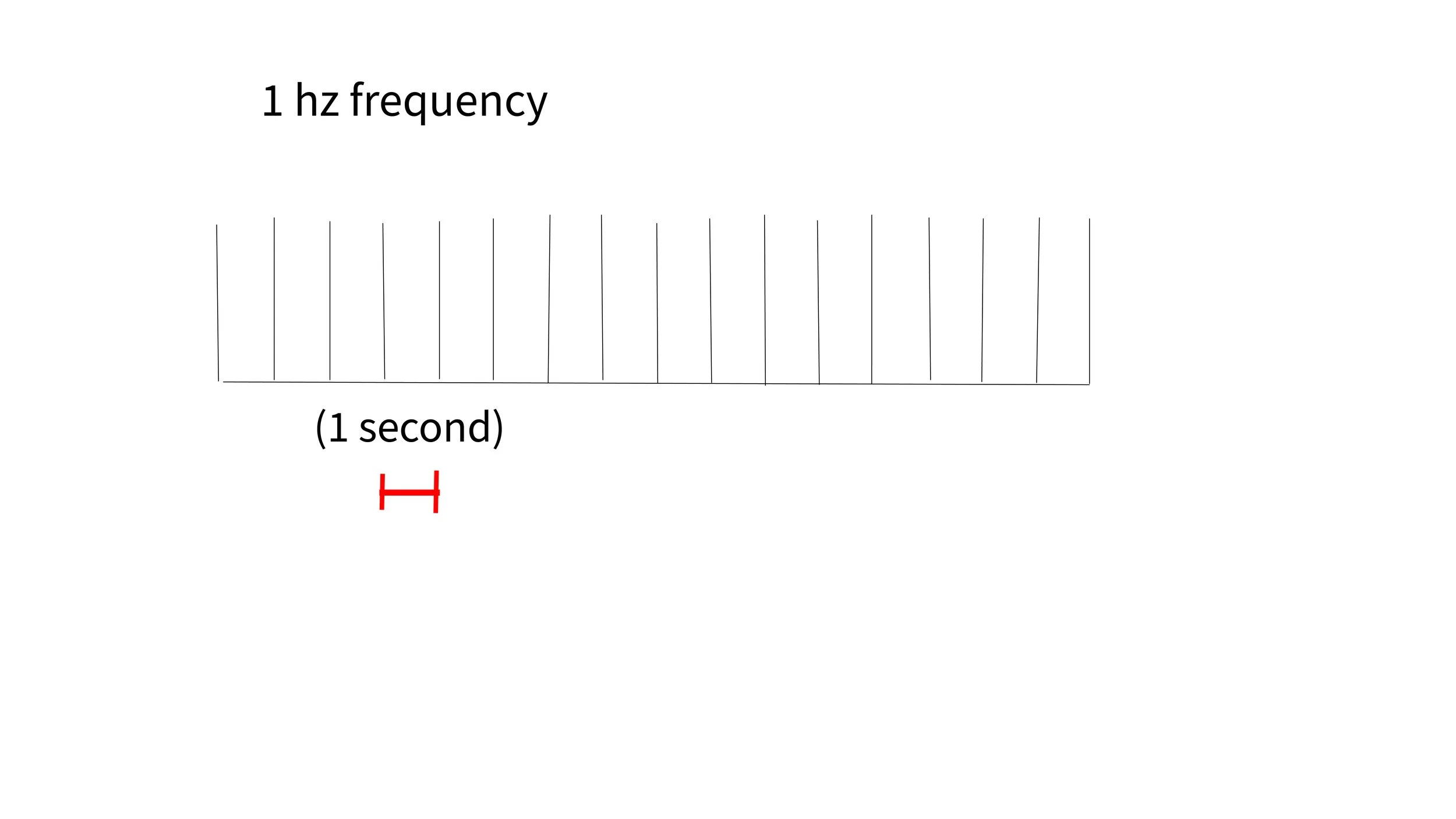
Low frequency: (1 pulse per second or less) Typically delivered over right Dosal Lateral Prefrontal Cortex (rDLPC). This tends to have an inhibitory effect on the targeted area.
A typical treatment with low frequency right sided DLPC targeting will have a frequency of 1 hz (1 pulse per second) or less for 15-20 minutes for a total of 900 -1200 pulses per treatment session.
-
Deep TMS: Not all TMS machines can do “deep” TMS. This involves an “H” coil which is different than a figure eight coil. This creates a slightly larger magnetic field that is able to penetrate deeper into the brain. The pulse parameters tend to be similar to rTMS as noted above. The benefits are that it can effect deeper structures but the area affected is much larger and thus (less targeted) compared to TMS using a figure 8 coil. An H coil can be used for MDD, OCD and even smoking cessation treatment. There is some evidence that it is less tolerated compared to other types of TMS. There was however a study by Filipcic et al. 2019 that compared regular “cortical” rTMS to “deep” TMS. The remission rates in the two groups were comparable but the response rates were higher in the deep TMS group (67%) vs. (44%) in the rTMS group.
-
iTBS: This stands for intermittent theta burst. Intermittent Theta burst TMS is a specific frequency of stimulation which is comprised of 50 hz frequency triplet bursts firing at a frequency of 5 hz for 2 seconds. This equals 30 pulses in a 2 second period. This is typically followed by an 8 second pause before the next train. (hence the term intermittent) The goal is to get 600 pulses per treatment. Obviously this cuts down the treatment time. A typical treatment duration is around 3.5 minutes.
Theta is a brain wave frequency between 4-7 hz. It is thought to be the frequency at which different parts of the brain communicate with each other. It is thought that treating at this frequency may help tune into the brains natural frequencies.
Theta burst TMS is a specific rhythm characterized by 3 pulse bursts at a rate of 50 hz. Those triplet pulses at 50 hz occur at a frequency of 5 hz. This occurs for 2 seconds in duration with an 8 second pause before the next train. This is referred to as intermittent theta pulse. There is also continuous theta burst which would continue without pause.
Intermittent theta burst or iTBS is safe and just as effective as rTMS but it requires only 600 pulses which can be completed in around 3.5 minutes where as rTMS requires 3000 pulses per treatment and typically takes anywhere between 17 and 40 minutes to complete.
Bulteau et al. 2022 compared iTBS with rTMS. Response rates were 36.7% and 33.3%, and remission rates were 18.5% and 14.8%, in the iTBS and 10 Hz rTMS groups respectively. This paper concludes that iTBS is just as effective as rTMS and is probably more cost effective.
-
aTMS: aTMS stands for accelerated TMS. Accelerated TMS is any TMS protocol that involves more than one treatment session per day. A study by Caufield et al. 2022 reviewed 63 different studies with 43,837 different aTMS sessions with 1543 participants. This review looked at safety, side effects and efficacy. With accelerated programs the seizure rate was 0.0023% vs. 0.0075% with once daily TMS. The most common side effects listed with aTMS were headaches, fatigue and scalp discomfort. The efficacy was reviewed of aTMS in 23 depression studies which showed a response rate of 42.4% and a remission rate of 28.4%. The treatment protocols ranged from 2-10 sessions per day with 2-30 treatment days, 10-640 minutes between sessions for a total of 9-104 total sessions per patient. This paper stated, “qualitatively response rate tends to be higher with an increasing number of sessions per day, total sessions, and total pulses.”
-
SAINT or SNT: SAINT stands for Stanford accelerated intelligent Neuromodulation therapy. SNT stands for Stanford Neuromodulation Therapy. They are the same protocol just renamed for branding purposes.
Cole et al. 2020 (SAINT) was an open label, accelerated, fMRI targeted TMS study looking at efficacy and safety in patients with Major Depressive Disorder with an N of 22. All patients received baseline fMRI to target the region of the left DLPFC most anticorrelated with sgACC. All patients then received 10 minutes of iTBS with 50 minute breaks for a total of 10 treatments per day for a total of 5 days. (180 pulses per minutes with iTBS x 10 = 1800 pulses per 10 minute session x 10 sessions = 18,000 pulses per day x 5 days = 90,000 pulses for total treatment course) The study found 86.4% - 90.5% of patients achieved remission by end of day 5. Neuropsychological testing demonstrated no negative cognitive side effects
Cole et al. 2022 (SNT) was a randomized, double blind, comparison of the SAINT TMS protocol with sham TMS. The primary outcome measure was change in MADRS score from baseline to week 4 post treatment. With an n of 29, the mean percent reduction from baseline in MADRS score 4 weeks after treatment was 52.5% in the active treatment group and 11.1% in the sham treatment group. Secondary measures included remission and response rates. In the treatment group 86.7% met response criteria (a reduction ≥50% in MADRS score) vs. 26.7% in the sham group in atleast one of the 5 posttreament assessments. In the treatment group, 78.6% met remission criteria (a MADRS score ≤10) vs. 13.3% in the sham group in the 4 week follow up period.
Evidence
-
Schutter et al. 2010: This study was a meta analysis of studies that compared double blinded sham-controls to TMS for treatment of depression symptoms. This study showed “a significant overall weighted mean effect size, d=0.39 [95% confidence interval (CI) 0.25-0.54], for active treatment was observed (z=6.52, p<0.0001)".” The paper concluded that high frequency rTMS over the left DLPFC was better than sham TMS. They commented that the effect size was compared to antidepressant medications.
Dunner et al. 2014: This study was a multisite, naturalistic, observational study looking at effectiveness of TMS in patients with Major Depressive Disorder who did not benefit from medication management. 257 patients completed a course of acute TMS treatment and consented to follow-up over 52 weeks. Assessments were obtained at 3, 6, 9, and 12 months with Clinical Global Impressions- Severity of illness scale, Inventory of Depression Symptoms- Self Report, 9-Item Patient Health Questioniare. 53% of all patients met remission or response criteria after the acute series. Among 120 patients who met IDS-SR response or remission criteria at the end of acute treatment, 75 (62.5%) continued to meet response criteria throughout long-term follow-up.(1 year) After the first month, when the majority of acute TMS tapering was completed, 93 patients (36.2%) received reintroduction of TMS. In this group, the mean (SD) number of TMS treatment days was 16.2. The conclusions of this study were that TMS demonstrated a statistically and clinically meaningful durability of acute benefit over 12 months of follow-up.
Senova et al 2019: This study was a systematic review and meta analysis of depression outcomes 3 months (732 patients), 6 months ( 695 patients) and 12 months (247 patients) after a course of TMS. Among initial responders, 66.5% sustained response at 3 months following treatment (95% CI = 57.1-74.8%, I2 = 27.6%), 52.9% at 6 months following TMS (95% CI = 40.3-65%, I2 = 0%), and 46.3% at 12 months (95% CI = 32.6-60.7%, I2 = 0%) The study concluded that “rTMS is a durable treatment for depression, with sustained responder rates of 50% up to 1 year after a successful induction course of treatment. Maintenance treatment may enhance the durability of the antidepressant effects of rTMS, and should be considered in clinical practice, as well as systematically explored in future clinical trials.”
Sackeim et al. 2020: This study was an evaluation on the registry data of 7759 patients in 103 practice sites who had a diagnosis of MDD and received a course of TMS. 5010 patients were included in an intent-to-treat (ITT) analysis. Response (58-83%) and remission (28-62%) rates were notably high across self-report and clinician-administered assessments. The paper concluded, “Strong efficacy and the low side effect and medical risk profile suggest that TMS be evaluated as a first-line treatment for MDD. The findings derive from the largest registry of clinical outcomes in MDD for any treatment.”
Via et al. 2023: This study looked at, “19 randomized double-blinded sham-controlled studies were included for quantitative analysis for response (n = 854 patients) and 9 studies for remission (n = 551 patients). The risk ratio (RR) for response and remission are 2.25 and 2.78, respectively for patients after two treatment failures using rTMS as add-on treatment compared to standard pharmacotherapy. Cochrane’s Q test showed no significant heterogeneity. No publication bias was detected.” 9 of the studies had remissions rates which were 35.71% in the active rTMS group and 8.37% in the sham rTMS group.
How does low frequency rTMS compare to traditional left-sided high frequency TMS?
The data suggest right sided, low-frequency, rTMS is just as effective as traditional left sided, high frequency TMS. It may have less side effects and have a lower risk of seizure.
Berlim et al 2013: This was a meta-anaylsis of randomized, double-blind, sham controlled trials investigating the effectiveness of low frequency right sided r TMS. This study included 8 RCT studies with an n of 263. This study showed 34.6% of those receiving low frequency, right sided rTMS met criteria for remission. 9.7% in the sham group met remission criteria.(OR=3.35; 95% CI=1.4–8.02; p=0.007) This study also demonstrated 38.2% of the active group met response criteria vs. 15.1% in the sham group. (OR=4.76; 95% CI=2.13–10.64; p<0.0001). The study concluded, “LF-rTMS is a promising treatment for MD, as it provides clinically meaningful benefits that are comparable to those of standard antidepressants and high-frequency rTMS. Furthermore, LF-rTMS seems to be an acceptable intervention for depressed subjects.”
Chen et al 2013: This was a systematic review and meta-analysis of 8 randomized studies comparing high frequency (HF) left sided r TMS with low frequency (LF) right sided r TMS. There was an N of 249. The two treatments were comparable. (odds ratio (OR) = 1.15; 95% confidence interval = 0.65-2.03).The study concluded, “The pooled examination demonstrated that both rTMS methods were equally effective therapies for MDD. However, considering that LF right-sided rTMS produces fewer side effects and is more protective against seizures, its clinical applicability shows greater promise and should be explored further.”
How long does TMS last?
Like any other psychiatric treatment, TMS is not a cure for depression. Individual results can vary from patient to patient. There is no good way to predict whether or not someone will respond to TMS let alone how long the improvement will last. Some the data below may be helpful in answering some of these questions.
Per the Dunner et al. 2014 study, 53% of patients met criteria for response/remission. 36.2% of those patients received some sort of taper after their acute series of 36 treatments. At 1 year follow up 62.5% of those that responded or remitted still met criteria fo response. In other words 12 months after starting TMS, 33% of all patients in this study continued to meet response criteria. It is confounded by some of the patients receiving more than a standard course of 36 treatments.
Per the Senova et al 2019study, among initial responders, 66.5% sustained response at 3 months following treatment, 52.9% at 6 months and 46.3% at 12 months. The study concluded that “rTMS is a durable treatment for depression, with sustained responder rates of 50% up to 1 year after a successful induction course of treatment. Maintenance treatment may enhance the durability of the antidepressant effects of rTMS, and should be considered in clinical practice, as well as systematically explored in future clinical trials.”
-
Carmi et al. 2019: This study was a multi-center, randomized, double-blind, sham controlled trial comparing deep TMS with sham TMS for the treatment of OCD. Both treatment and sham groups had 29 treatment sessions with 5 treatment days per week for 5 weeks then 4 treatment days the 6th week with one day for assessments. The primary outcome measure was change in YBOC score 6 weeks after treatment initiation. The active treatment group received 20 hz frequency dTMS at 100% MT with 2 second pulse trains and 20 second intervals between trains. This totaled 50 trains or 2000 pulses per treatment session. The primary outcome measure was change in YBOC which was -6 points for treatment group (95% CI 4.0-8.1) and -3.3 points for the sham group (95% CI 1.2, 5.3). Effect size was 0.69. Secondary measures included response rate which was 45.2% in the treatment group and 17.8% in the sham group.
Gersner et al. 2019: This paper looked at two different studies, one of a multicenter study where patients received active dTMS (n=42) and an open label sample (n = 26). All patients received YBOC assessments weekly. The YBOC scores reduced by 8.6 + or - 0.8 or a 30.1% reduction from baseline through week 6 of treatments. (P < 0.001) 60.3% had a partial response as defined by atleast 20 percent decrease in YBOCS. 47.1% of patients met response criteria which is atleast a 30 % reduction in YBOCS.
-
Cox et al. 2022: This paper is a systematic review and meta-analysis of patients with either Generalized Anxiety Disorder or Panic Disorder receiving rTMS and what effect the rTMS had on their symptom rating scales. The paper looked at 13 different studies with 677 patients total, 404 of which were in the treatment group and 273 did not receive rTMS) Per Cox et al, “In GAD patients with or without any comorbidities, rTMS therapy demonstrated significant improvements in anxiety (SMD = 1.45; P < .001) and depression (SMD = 1.65; P < .001) scores regardless of rTMS parameters. Overall anxiety (SMD = 0.24; P = .48) and panic severity (SMD = 1.19; P = .054) scores did not significantly improve after rTMS therapy in patients with PD.” Therefor, the study concludes that rTMS can be a safe and effective treatment for improving anxiety scores in GAD.
How does TMS work for depression?
The exact mechanism by which TMS alleviates symptoms of depression remains elusive. There is some evidence that it possibly restores aberrantly functioning neurocircuits that are implicated in depression.
Interesting papers worth mentioning:
Weigand et al 2018 showed “functional connectivity between an individual's rTMS cortical target (DLPC) and the subgenual cingulate predicts antidepressant response.”
Per Furtado et al. 2013, neuroimaging showed increased in left amygdala volume in those patients with symptom improvement and non-responders showed a decline in left hippocampus volumes. The study stated, “rTMS may promote neurogenesis or other effects that favor neuronal plasticity.”
Peters et al. 2016: This paper describes the neurological underpinnings of depression. It goes into vivid detail about the CSTS (cortico-striatal-thalamic-cortical) loop. It also describes some key functional networks.
McTeague et al. 2017 investigated common neural circuits that are disrupted in a variety of psychiatric conditions. The study compared 5,728 healthy controls with 5,493 patients with different psychiatric diagnosis including Major Depressive Disordfer, Bipolar DIsorder, Schizophrenia, Anxiety disorders and Substance use disorders. The study showed, “abnormal activation was evident in the left prefrontal cortex as well as the anterior insula, the right ventrolateral prefrontal cortex, the right intraparietal sulcus, and the midcingulate/presupplementary motor area. Disruption was also observed in a more anterior cluster in the dorsal cingulate cortex, which overlapped with a network of structural perturbation that the authors previously reported in a transdiagnostic meta-analysis of gray matter volume.” The paper then concluded, “the anterior-cingulo-insular or "salience network" demonstrated to be transdiagnostically vulnerable to gray matter reduction. Thus, networks intrinsic to adaptive, flexible cognition are vulnerable to broad-spectrum psychopathology.”
References:
Schutter DJ. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychol Med. 2009 Jan;39(1):65-75. doi: 10.1017/S0033291708003462. Epub 2008 Apr 30. PMID: 18447962.
Dunner DL, Aaronson ST, Sackeim HA, Janicak PG, Carpenter LL, Boyadjis T, Brock DG, Bonneh-Barkay D, Cook IA, Lanocha K, Solvason HB, Demitrack MA. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014 Dec;75(12):1394-401. doi: 10.4088/JCP.13m08977. PMID: 25271871.
O'Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackeim HA. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007 Dec 1;62(11):1208-16. doi: 10.1016/j.biopsych.2007.01.018. Epub 2007 Jun 14. PMID: 17573044.
Sackeim HA, Aaronson ST, Carpenter LL, Hutton TM, Mina M, Pages K, Verdoliva S, West WS. Clinical outcomes in a large registry of patients with major depressive disorder treated with Transcranial Magnetic Stimulation. J Affect Disord. 2020 Dec 1;277:65-74. doi: 10.1016/j.jad.2020.08.005. Epub 2020 Aug 7. PMID: 32799106.
George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, Anderson B, Nahas Z, Bulow P, Zarkowski P, Holtzheimer PE 3rd, Schwartz T, Sackeim HA. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010 May;67(5):507-16. doi: 10.1001/archgenpsychiatry.2010.46. PMID: 20439832.
Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, Nejad R, Pankow H, Choi E, Aaron H, Espil FM, Pannu J, Xiao X, Duvio D, Solvason HB, Hawkins J, Guerra A, Jo B, Raj KS, Phillips AL, Barmak F, Bishop JH, Coetzee JP, DeBattista C, Keller J, Schatzberg AF, Sudheimer KD, Williams NR. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. Am J Psychiatry. 2020 Aug 1;177(8):716-726. doi: 10.1176/appi.ajp.2019.19070720. Epub 2020 Apr 7. PMID: 32252538
Berlim MT, Van den Eynde F, Daskalakis ZJ. Efficacy and acceptability of high frequency repetitive transcranial magnetic stimulation (rTMS) versus electroconvulsive therapy (ECT) for major depression: a systematic review and meta-analysis of randomized trials. Depress Anxiety. 2013 Jul;30(7):614-23. doi: 10.1002/da.22060. Epub 2013 Jan 24. PMID: 23349112.
Bulteau S, Laurin A, Pere M, Fayet G, Thomas-Ollivier V, Deschamps T, Auffray-Calvier E, Bukowski N, Vanelle JM, Sébille V, Sauvaget A. Intermittent theta burst stimulation (iTBS) versus 10 Hz high-frequency repetitive transcranial magnetic stimulation (rTMS) to alleviate treatment-resistant unipolar depression: A randomized controlled trial (THETA-DEP). Brain Stimul. 2022 May-Jun;15(3):870-880. doi: 10.1016/j.brs.2022.05.011. Epub 2022 May 21. PMID: 35609816.
Senova S, Cotovio G, Pascual-Leone A, Oliveira-Maia AJ. Durability of antidepressant response to repetitive transcranial magnetic stimulation: Systematic review and meta-analysis. Brain Stimul. 2019 Jan-Feb;12(1):119-128. doi: 10.1016/j.brs.2018.10.001. Epub 2018 Oct 2. PMID: 30344109.
Schutter DJ. Quantitative review of the efficacy of slow-frequency magnetic brain stimulation in major depressive disorder. Psychol Med. 2010 Nov;40(11):1789-95. doi: 10.1017/S003329171000005X. Epub 2010 Jan 27. PMID: 20102670.
Schimmelpfennig J, Topczewski J, Zajkowski W, Jankowiak-Siuda K. The role of the salience network in cognitive and affective deficits. Front Hum Neurosci. 2023 Mar 20;17:1133367. doi: 10.3389/fnhum.2023.1133367. PMID: 37020493; PMCID: PMC10067884.
Iseger TA, Arns M, Downar J, Blumberger DM, Daskalakis ZJ, Vila-Rodriguez F. Cardiovascular differences between sham and active iTBS related to treatment response in MDD. Brain Stimul. 2020 Jan-Feb;13(1):167-174. doi: 10.1016/j.brs.2019.09.016. Epub 2019 Oct 9. PMID: 31629693.
Furtado CP, Hoy KE, Maller JJ, Savage G, Daskalakis ZJ, Fitzgerald PB. An investigation of medial temporal lobe changes and cognition following antidepressant response: a prospective rTMS study. Brain Stimul. 2013 May;6(3):346-54. doi: 10.1016/j.brs.2012.06.006. Epub 2012 Jul 6. PMID: 22784443.
Peters SK, Dunlop K, Downar J. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front Syst Neurosci. 2016 Dec 27;10:104. doi: 10.3389/fnsys.2016.00104. PMID: 28082874; PMCID: PMC5187454.
Filipčić I, Šimunović Filipčić I, Milovac Ž, Sučić S, Gajšak T, Ivezić E, Bašić S, Bajić Ž, Heilig M. Efficacy of repetitive transcranial magnetic stimulation using a figure-8-coil or an H1-Coil in treatment of major depressive disorder; A randomized clinical trial. J Psychiatr Res. 2019 Jul;114:113-119. doi: 10.1016/j.jpsychires.2019.04.020. Epub 2019 Apr 26. PMID: 31059991.
Caulfield KA, Fleischmann HH, George MS, McTeague LM. A transdiagnostic review of safety, efficacy, and parameter space in accelerated transcranial magnetic stimulation. J Psychiatr Res. 2022 Aug;152:384-396. doi: 10.1016/j.jpsychires.2022.06.038. Epub 2022 Jun 28. PMID: 35816982; PMCID: PMC10029148.
Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, Nejad R, Pankow H, Choi E, Aaron H, Espil FM, Pannu J, Xiao X, Duvio D, Solvason HB, Hawkins J, Guerra A, Jo B, Raj KS, Phillips AL, Barmak F, Bishop JH, Coetzee JP, DeBattista C, Keller J, Schatzberg AF, Sudheimer KD, Williams NR. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. Am J Psychiatry. 2020 Aug 1;177(8):716-726. doi: 10.1176/appi.ajp.2019.19070720. Epub 2020 Apr 7. PMID: 32252538.
Cole EJ, Phillips AL, Bentzley BS, Stimpson KH, Nejad R, Barmak F, Veerapal C, Khan N, Cherian K, Felber E, Brown R, Choi E, King S, Pankow H, Bishop JH, Azeez A, Coetzee J, Rapier R, Odenwald N, Carreon D, Hawkins J, Chang M, Keller J, Raj K, DeBattista C, Jo B, Espil FM, Schatzberg AF, Sudheimer KD, Williams NR. Stanford Neuromodulation Therapy (SNT): A Double-Blind Randomized Controlled Trial. Am J Psychiatry. 2022 Feb;179(2):132-141. doi: 10.1176/appi.ajp.2021.20101429. Epub 2021 Oct 29. PMID: 34711062.
Weigand A, Horn A, Caballero R, Cooke D, Stern AP, Taylor SF, Press D, Pascual-Leone A, Fox MD. Prospective Validation That Subgenual Connectivity Predicts Antidepressant Efficacy of Transcranial Magnetic Stimulation Sites. Biol Psychiatry. 2018 Jul 1;84(1):28-37. doi: 10.1016/j.biopsych.2017.10.028. Epub 2017 Nov 10. PMID: 29274805; PMCID: PMC6091227.
Berlim MT, Van den Eynde F, Jeff Daskalakis Z. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013 Mar;38(4):543-51. doi: 10.1038/npp.2012.237. Epub 2012 Nov 19. PMID: 23249815; PMCID: PMC3572468.
Chen J, Zhou C, Wu B, Wang Y, Li Q, Wei Y, Yang D, Mu J, Zhu D, Zou D, Xie P. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. 2013 Dec 30;210(3):1260-4. doi: 10.1016/j.psychres.2013.09.007. Epub 2013 Oct 7. PMID: 24113125.
Deng ZD, Lisanby SH, Peterchev AV. Coil design considerations for deep transcranial magnetic stimulation. Clin Neurophysiol. 2014 Jun;125(6):1202-12. doi: 10.1016/j.clinph.2013.11.038. Epub 2013 Dec 22. PMID: 24411523; PMCID: PMC4020988.
Zhang M, Wang R, Luo X, Zhang S, Zhong X, Ning Y, Zhang B. Repetitive Transcranial Magnetic Stimulation Target Location Methods for Depression. Front Neurosci. 2021 Sep 9;15:695423. doi: 10.3389/fnins.2021.695423. PMID: 34566561; PMCID: PMC8458642.
McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of Common Neural Circuit Disruptions in Cognitive Control Across Psychiatric Disorders. Am J Psychiatry. 2017 Jul 1;174(7):676-685. doi: 10.1176/appi.ajp.2017.16040400. Epub 2017 Mar 21. PMID: 28320224; PMCID: PMC5543416.
Cox J, Thakur B, Alvarado L, Shokar N, Thompson PM, Dwivedi AK. Repetitive transcranial magnetic stimulation for generalized anxiety and panic disorders: A systematic review and meta-analysis. Ann Clin Psychiatry. 2022 May;34(2):e2-e24. doi: 10.12788/acp.0050. PMID: 35550035.
Carmi L, Tendler A, Bystritsky A, Hollander E, Blumberger DM, Daskalakis J, Ward H, Lapidus K, Goodman W, Casuto L, Feifel D, Barnea-Ygael N, Roth Y, Zangen A, Zohar J. Efficacy and Safety of Deep Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: A Prospective Multicenter Randomized Double-Blind Placebo-Controlled Trial. Am J Psychiatry. 2019 Nov 1;176(11):931-938. doi: 10.1176/appi.ajp.2019.18101180. Epub 2019 May 21. PMID: 31109199.
Li CT, Cheng CM, Chen MH, Juan CH, Tu PC, Bai YM, Jeng JS, Lin WC, Tsai SJ, Su TP. Antidepressant Efficacy of Prolonged Intermittent Theta Burst Stimulation Monotherapy for Recurrent Depression and Comparison of Methods for Coil Positioning: A Randomized, Double-Blind, Sham-Controlled Study. Biol Psychiatry. 2020 Mar 1;87(5):443-450. doi: 10.1016/j.biopsych.2019.07.031. Epub 2019 Aug 9. PMID: 31563272.
Gersner, R., Sisko, E. & Tendler, A. How much can patients expect to improve with six weeks of deep TMS for OCD? Brain Stimulation 12, 525 (2019