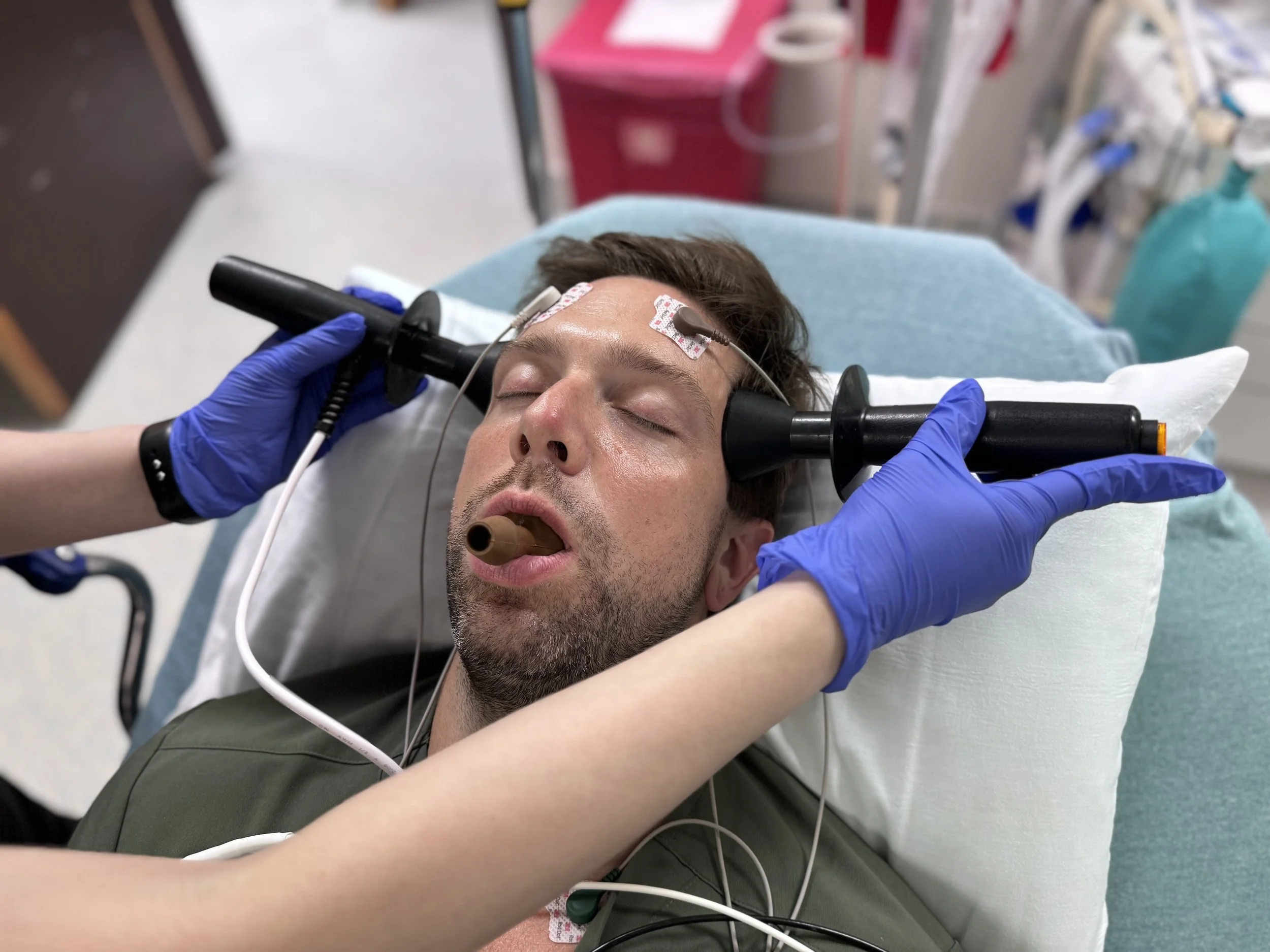
ECT Information for Patients
-
ECT stands fo Electroconvulsive Therapy. ECT is a medical procedure done under general anesthesia which means the patient is completely asleep during the procedure. After the patient is asleep, electrodes are placed on the head to send a small electrical current through the brain that induces a short seizure. This seizure causes physiologic changes in the brain that can lead to clinical improvement.
The seizure typically lasts between 20 seconds and 2 minutes. The entire treatment itself usually lasts only about 10-15 minutes. The longest part of the treamtment is getting the patient ready and then monitoring the patient after the treatment while they wake up and recover enough to be safely discharged.
-
Treatment Resistant Major Depressive Disorder. (Failure to respond to at least 2 adequate trials of an antidepressant with or without psychotherapy)
Major Depressive Disorder (with suicidality or extreme functional impairment)
Major Depressive Disorder with psychotic feature
Catatonia
Bipolar Depression
Schizophrenia
Schizaffetive Disorder
-
Common side effects from ECT include:
Headache
Nausea
Muscle Aches
Memory Loss
Feeling worn out on treatment day
-
Overall, ECT is considered a safe treatment. The overall risk of death is about 1 in 70,000 patients according to Watts et al. 2011.
There is the risk of death, heart attack, stroke, anoxic injury, status epileptics (a seizure that keeps going), aspiration etc. These risks can change based on your age, comorbid medical issues and how well those medical issues are managed.
It’s important to have a comprehensive and thorough psychaitric as well as medical evaluation prior to ECT. A discussion with your psychiatrist about the risks/benefits and alternatives to ECT are required before starting treatment.
While there are risks involved with ECT treatment there are also risks in under treating the patient.
-
ECT causes several physiologic changes in the brain that lead to clinical improvement.
For more information please see below section under Mechanism of Action.
-
ECT is considered the most effective treatment for MDD.
However, it is a big production to get driven to and from ECT three times a week after you are put under general anesthesia and given a seizure which in most patients causes some memory loss. In other words, ECT is way more intensive and risky than talking to a therapist once a week!
Just because something is the “most effective” doesn’t mean it is necessarily “the right treatment” for every patient.
Opinion Alert!
In my opinion, a patient centered approach is paramount to the ECT treatment process. This includes shared decision making where the psychiatrist (me) is helping you the patient (and family) decide what treatment makes the most sense based on the risks/benefits and alternatives to ECT.
-
ECT Information for Clinicians
-
It is common to start ECT three times per week. Typically this is done on a Monday, Wednesday and Friday. This is referred to as an Index course.
Once the patient, their family and the treating physician agree the majority of symptoms have improved, the patient may begin to taper the treatments. The ECT treatments after an index course are called continuation. A typical continuation course can be twice weekly followed by weekly treatments etc. The goal is to slowly increase the interval in between treatments (decreasing the frequency) while monitoring for side effects and any relapse of symptoms. Treatments that are more than 6 months after an index course are referred to as “maintenance.” Maintenance ECT is an effort to prevent a recurrence symptoms.
Summary:
Index course: Three times per week. An average amount of treatments can be 9-12 treatments. This of course varies based on the individual patient.
Continuation: Period after index course for 6 months that is meant to prevent a relapse of symptoms.
Maintenance: Period 6 months or more after an Index course that helps prevent a recurrence of symptoms.
-
Think of the charge as the dose of ECT. Charge is the number of electrons going through the brain. The higher the dose, the more potential for benefit but also the more RISK of side effects (memory issues). Below is an example of a dosing titration schedule for Bilateral and right unilateral ECT. This is the schedule I use in my practice and the schedule that was utilized at the University of Michigan.
-
Common positions for lead placement includes Bilateral (Bitemporal), Right Unilateral or Bifrontal Placement. According to the meta-analysis that looked at Bitemporal (Bilateral) ECT vs. RUL ECT by Kolshus et al. 2017, RUL worked just as well for treating depression but has some advantages for time to reorientation and retrograde memory.
Many patients respond well to RUL treatment with an ultra brief pulse width (0.3-0.37ms). The benefit of this approach is clinical effect with less memory side effects. There are some patients that dont respond to RUL ultra brief in which case the patient is switched to bilateral treatments. Bilateral treatment has the benefits of working faster for some patients at the expense of possibly leading to more memory issues. Reasons to start with bilateral ECT for a patient may include catatonia, neuro-vegetation, psychosis or severe suicidality.
-
What medications are used in treatment?
Most patients receive several medications for each ECT treatment. Most are administered via an IV. The medications typically include an anesthetic, paralytic, analgesic, anti-emetic and an anti-muscarinic. The medications and dosages are individualized to the patients body mass and specific requirements.
Common medications can include:
Anesthetic:
Brevital (1 mg/kg range)
Etomidate (0.2 - 0.3 mg/kg range)
Paralytic:
Succinylcholine (1 mg/kg range)
Rocuronium (0.5 - 1mg/kg range)
Analgesic:
Tylenol 500 mg -1000 mg PO
Toradol 15 - 30 mg
Anti-emetic:
Zofran 4 - 8 mg
Phenergan 6.25 -12.5 mg
Miscellaneous:
glycopyrrolate (0.1 - 0.4 mg) for bradycardia and salivation
labetalol (5 - 10 mg) for hypertension
ketamine (15 - 60 mg) for anesthetic, analgesia and lowering seizure threshold
flumazenil (0.25 - 1 mg) for benzodiazepine reversal
caffeine (125 - 500 mg) for increased seizure duration
versed (1 - 4 mg) for postictal agitation/anxiety or prolonged seizure
sugammadex for rocuronium reversal (weight based)
-
Monoamine Hypothesis: Similar to antidepressant medications, ECT causes changes in the relative amounts of serotonin, norepinephrine and dopamine that is utilized in the synapses which is thought to lead to adaptive changes in receptors and thus functioning of the circuits associated with those receptors.
Genetic Changes: There appear to be many genetic changes that occur following ECT in animal models. Dyrviget al. 2014 stated “We find significant increases for c-Fos, Egr1, Neuritin 1 (Nrn 1), Bdnf, Snap29, Synaptotagmin III (Syt 3), Synapsin I (Syn 1), and Psd95.”
Neurotrophic Factors: Brain Derived Neurotrophic Factor (BDNF) was found to be elevated in patients after ECT according to Brunoni et al. 2014. It is important to remember that there has been no established correlation found between clinical improvement with ECT and BDNF levels.
Vascular Endothelial Growth Factor (VEGF) was found to be increased 1 month after patients received ECT. Minelli et al. 2011
Hypothalmic- Pituitary- Adrenal axis (HPA). Patients with depression tend to show elevated cortisol levels. ECT appears to help restore cortisol to normal levels although it is unclear if could be a direct cause of improvement or a result of some other mechanism. Hasket et al. 2014
Functional Connectivity: There is some evidence that ECT leads to physiological changes that better enable different networks in the brain to communicate more effectively. Beal et al. 2012 demonstrated the anterior cingulate cortex (aCC) showed increased connectivity to the right dorsal lateral prefrontal cortex (rDLPC) and the Posterior Cingulate Cortex (PCC). This paper is also interesting in that there appears to be a correlation in the increased connectivity and clinical improvement. It’s important to pointout there are other studies that do not show a positive correlation between increased connectivity and clinical improvement. (Abbott et al., 2013; Li et al., 2013, Posner et al., 2013)
GABA Hypothesis: ECT appears to increase GABA in patients receiving ECT. Esel et al. 2008
-
According to the article by Anderson 2021 there are 11 RCT’s comparing sham ECT to real ECT and 5 meta analysis. In the article he states “despite their methodological differences, there is a consistent direction in the findings of the RCTs of at least a numerical benefit for ECT over sham ECT at the end of the comparison treatment period, and all meta-analyses report a significant pooled result in favor of ECT irrespective of the combination of studies they include.”
UK ECT group 2003: Reviewed efficacy and safety of ECT vs. Sham ECT in RCT’s in addition to ECT vs. medications and different types of ECT. That paper concluded, “Real ECT was significantly more effective than simulated ECT (six trials, 256 patients, standardised effect size [SES] -0.91, 95% CI -1.27 to -0.54). Treatment with ECT was significantly more effective than pharmacotherapy (18 trials, 1144 participants, SES -0.80, 95% CI -1.29 to -0.29). Bilateral ECT was more effective than unipolar ECT (22 trials, 1408 participants, SES -0.32, 95% CI -0.46 to -0.19).”
Berlim et al. 2013: A systematic review and meta-analysis (n = 294) comparing ECT with rTMS. This analysis showed a higher rate of remission for ECT (52%) as compared to rTMS (34%). OR = 0.46 [95% CI: 0.22 to 0.96; z = -2.06; p = 0.04]
Ren et al. 2014: A meta-analysis ( n = 429) comparing ECT with rTMS. This analysis showed a higher rate of response with ECT (52.9%) as compared to rTMS (33.6%). RR = 1.38, P = 0.006. The study also showed a higher rate of response with ECT (64.4%) as compared to rTMS ( 48.7%). RR = 1.41, p = 0.03.
Sackeim et al. 1987: Randomized control trial (n = 52) showed that low dose bilateral ECT had a higher response (70%) when compared to low dose right unilateral ECT (28%).
Sackeim et al. 1993: Randomized control trial (n = 96) comparing threshold vs. 2.5x threshold ECT in either Bilateral or Right Unilateral placement. The response rates were 63% in the 2.5x threshold bilateral group, 65% in the threshold bilateral group, 43% in the 2.5x threshold right unilateral group and 17 % in the threshold right unilateral group.
Sackeim et al. 2000: Randomized controlled trial (n = 80) comparing response rates of Bilateral placement 150 % above seizure threshold (65% response) and right unilateral placement 500% above seizure threshold (65% response) with right unilateral placement dosed at 50% above seizure threshold (35% response) and 150% above seizure threshold (30%response).
McCall et al. 2000: Randomized control trial comparing fixed dose of 403 mC (higher dose) right unilateral placement with 2.25 x seizure threshold with right unilateral placement. The response rates were 67% vs. 39% respectively.
Semkovska et al 2010: “Cognitive abnormalities associated with ECT are mainly limited to the first 3 days posttreatment. Pretreatment functioning levels are subsequently recovered. After 15 days, processing speed, working memory, anterograde memory, and some aspects of executive function improve beyond baseline levels.”
Rhee et al. 2022: A systematic review and meta-analysis of 6 clinical trials (5 of which were randomized clinical trials), composed of 340 patients, who either received ECT (n = 162) or ketamine (n = 178). The SMD per this paper was -0.69 (95% CI, -0.89 to -0.48; Cochran Q, P = 0.15; I^2 = 39%). This study concluded “ECT may be superior to ketamine for improving symptom severity in the acute phase, but treatment options should be individualized and patient-centered.”
-
Electricity is simply the flow of charged ions (electrons). The electricity that comes out of the wall in most homes is a sine wave. (see diagram to the right) This is the wave form of electricity that used to be used in ECT. Advances in ECT lead to the development of a square wave stimulation. (See diagram to the right)
The optimal pulse width for depolarization of a neuron is approximately 0.1 to 0.2 ms according to Geddes et al. 1987.
The advantages of square wave stimulation include a lesser amount of charge (electrons) that are used. Studies show that square wave administration is just as effective as sine wave but helps to cut down on the cognitive side effects that are common with ECT treatment. Weiner et al. 1986
Most modern ECT machines generate a biphasic wave form. This in reality doubles the frequency. As such this needs to be considered when calculating the charge being delivered.
An ECT stimulation dose is measured in millicoulumbs.(abbreviated mC) This is a measure of charge. The charge (dose) is a function of the amplitude of the wave form, the frequency, the pulse width and the total duration of the stimulus. Think of the dose (aka charge) as measuring the area of the boxes and then counting the total number of boxes. That is the charge (dose) given during the stimulation. According to Kho et al. 2003, “No evidence was found for a superior speed of action of ECT or for a difference in efficacy between sine wave and brief pulse stimulation.”
Shortening the pulse width can help reduce the cognitive side effects associated with ECT. There were two randomized controlled trials that compared brief pulse to ultrabrief pulse ECT. Pisvejc et al. 1998 and Sackeim et al. 2008. Both studies showed ultrabrief (<0.5 ms) is just as effective as brief (0.5-2.0 ms). Per Sackeim et al. 2008, “The use of an ultrabrief stimulus markedly reduces adverse cognitive effects, and when coupled with markedly suprathreshold right unilateral ECT, also preserves efficacy.”
-
Coulomb is a unit of electrical charge. 1 Coulomb = 6.241 x 10^18 electrons
or
1 e (electron) = 1.602176634 x 10^-19 Coulombs
Charge = current x time
So charge is the number of electrons passing through a conductor over a certain period of time
Charge = frequency (x2 for biphasic waveform) x duration x pulse width x amplitude
As an example: A stimulus with a current of 800 mA (0.8 amps) x pulse width of 0.37 ms (0.00037 seconds) x a frequency of 120 (120 hz x 2 since the wave form is biphasic) x duration (8 seconds)
0.8 x 0.00037 x (120 x 2) x 8 = .568 C or 568 mC
Energy (Joules) = Charge (coulombs) x Time (seconds) x impedance (Ohms)
or
Energy (Joules) = current(amps)^2 x resistance (Ohms) x time (seconds)
Think of the time variable as the total duration of boxes. You are just adding them up. So many boxes (pulses per second) x pulse width (measured in milliseconds) x total duration of stimulation.
Continuing with the example above: A current with 0.8 amps (x 0.8) x 220 Ohms x (0.00037 x 240 x 8)
or
0.64 Amps x 220 Ohms x 0.7104 seconds = 100.02 Joules
Voltage = Energy/Charge or V = J/C
or
100.02 Joules/0.568 Coulombs = 176 Voltz
References:
UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003 Mar 8;361(9360):799-808. doi: 10.1016/S0140-6736(03)12705-5. PMID: 12642045.
Anderson IM. Electroconvulsive therapy (ECT) versus sham ECT for depression: do study limitations invalidate the evidence (and mean we should stop using ECT)? BJPsych Advances. 2021;27(5):285-291. doi:10.1192/bja.2021.23
Meechan CF, Laws KR, Young AH, McLoughlin DM, Jauhar S. A critique of narrative reviews of the evidence-base for ECT in depression. Epidemiology and Psychiatric Sciences. 2022;31:e10. doi:10.1017/S2045796021000731
Kolshus E, Jelovac A, McLoughlin DM. Bitemporal v. high-dose right unilateral electroconvulsive therapy for depression: a systematic review and meta-analysis of randomized controlled trials. Psychol Med. 2017 Feb;47(3):518-530. doi: 10.1017/S0033291716002737. Epub 2016 Oct 26. Erratum in: Psychol Med. 2017 Sep 18;:1-2. PMID: 27780482.
Ren J, Li H, Palaniyappan L, Liu H, Wang J, Li C, Rossini PM. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014 Jun 3;51:181-9. doi: 10.1016/j.pnpbp.2014.02.004. Epub 2014 Feb 18. PMID: 24556538.
Berlim MT, Van den Eynde F, Daskalakis ZJ. High-frequency repetitive transcranial magnetic stimulation accelerates and enhances the clinical response to antidepressants in major depression: a meta-analysis of randomized, double-blind, and sham-controlled trials. J Clin Psychiatry. 2013 Feb;74(2):e122-9. doi: 10.4088/JCP.12r07996. PMID: 23473357.
Sackeim HA, Prudic J, Devanand DP, Kiersky JE, Fitzsimons L, Moody BJ, McElhiney MC, Coleman EA, Settembrino JM. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med. 1993 Mar 25;328(12):839-46. doi: 10.1056/NEJM199303253281204. PMID: 8441428.
Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, Fitzsimons L, Moody BJ, Clark J. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000 May;57(5):425-34. doi: 10.1001/archpsyc.57.5.425. PMID: 10807482.
Sackeim HA, Decina P, Kanzler M, Kerr B, Malitz S. Effects of electrode placement on the efficacy of titrated, low-dose ECT. Am J Psychiatry. 1987 Nov;144(11):1449-55. doi: 10.1176/ajp.144.11.1449. PMID: 3314538.
McCall WV, Reboussin DM, Weiner RD, Sackeim HA. Titrated moderately suprathreshold vs fixed high-dose right unilateral electroconvulsive therapy: acute antidepressant and cognitive effects. Arch Gen Psychiatry. 2000 May;57(5):438-44. doi: 10.1001/archpsyc.57.5.438. PMID: 10807483.
Kho KH, van Vreeswijk MF, Simpson S, Zwinderman AH. A meta-analysis of electroconvulsive therapy efficacy in depression. J ECT. 2003 Sep;19(3):139-47. doi: 10.1097/00124509-200309000-00005. PMID: 12972983.
Pisvejc J, Hyrman V, Sikora J, Berankova A, Kobeda B, Auerova M, Sochorova V. A comparison of brief and ultrabrief pulse stimuli in unilateral ECT. J ECT. 1998 Jun;14(2):68-75. PMID: 9641801.
Sackeim HA, Prudic J, Nobler MS, Fitzsimons L, Lisanby SH, Payne N, Berman RM, Brakemeier EL, Perera T, Devanand DP. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008 Apr;1(2):71-83. doi: 10.1016/j.brs.2008.03.001. Erratum in: Brain Stimul. 2008 Jul;1(3):A2. PMID: 19756236; PMCID: PMC2742986.
Singh A, Kar SK. How Electroconvulsive Therapy Works?: Understanding the Neurobiological Mechanisms. Clin Psychopharmacol Neurosci. 2017 Aug 31;15(3):210-221. doi: 10.9758/cpn.2017.15.3.210. PMID: 28783929; PMCID: PMC5565084.
Ranck JB Jr. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975 Nov 21;98(3):417-40. doi: 10.1016/0006-8993(75)90364-9. PMID: 1102064.
Brunoni AR, Baeken C, Machado-Vieira R, Gattaz WF, Vanderhasselt MA. BDNF blood levels after electroconvulsive therapy in patients with mood disorders: a systematic review and meta-analysis. World J Biol Psychiatry. 2014 Jul;15(5):411-8. doi: 10.3109/15622975.2014.892633. Epub 2014 Mar 16. PMID: 24628093.
Haskett RF. Electroconvulsive therapy's mechanism of action: neuroendocrine hypotheses. J ECT. 2014 Jun;30(2):107-10. doi: 10.1097/YCT.0000000000000143. PMID: 24800689.
Beall EB, Malone DA, Dale RM, Muzina DJ, Koenig KA, Bhattacharrya PK, Jones SE, Phillips MD, Lowe MJ. Effects of electroconvulsive therapy on brain functional activation and connectivity in depression. J ECT. 2012 Dec;28(4):234-41. doi: 10.1097/YCT.0b013e31825ebcc7. PMID: 22820953.
Gudayol-Ferré E, Peró-Cebollero M, González-Garrido AA, Guàrdia-Olmos J. Changes in brain connectivity related to the treatment of depression measured through fMRI: a systematic review. Front Hum Neurosci. 2015 Nov 3;9:582. doi: 10.3389/fnhum.2015.00582. PMID: 26578927; PMCID: PMC4630287.
Esel E, Kose K, Hacimusalar Y, Ozsoy S, Kula M, Candan Z, Turan T. The effects of electroconvulsive therapy on GABAergic function in major depressive patients. J ECT. 2008 Sep;24(3):224-8. doi: 10.1097/YCT.0b013e31815cbaa1. PMID: 18562944.
Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010 Sep 15;68(6):568-77. doi: 10.1016/j.biopsych.2010.06.009. Epub 2010 Jul 31. PMID: 20673880.
Watts BV, Groft A, Bagian JP, Mills PD. An examination of mortality and other adverse events related to electroconvulsive therapy using a national adverse event report system. J ECT. 2011 Jun;27(2):105-8. doi: 10.1097/YCT.0b013e3181f6d17f. PMID: 20966769.
Espinoza RT, Kellner CH. Electroconvulsive Therapy. N Engl J Med. 2022 Feb 17;386(7):667-672. doi: 10.1056/NEJMra2034954. PMID: 35172057.